Albumin: an Exceptional Biomaterial
Biomaterials are defined as natural or synthetic substances engineered to interact with biological systems in order to restore, support, or enhance physiological functions. Their value lies in a combination of properties such as biocompatibility, stability, structural adaptability, and the ability to support tissue regeneration, healing, or controlled therapeutic delivery.
Within this broad category, natural biomatters occupy a special position because they combine intrinsic compatibility with the human body and physicochemical features that can be engineered into functional architectures.
Among natural biomatters, albumin stands out as uniquely versatile. As the most abundant protein in human plasma, albumin is highly stable, soluble, non-immunogenic, and easily purified.
It exhibits rare physicochemical characteristics: a capacity for controlled denaturation leading to intermolecular crosslinking, an ability to transition from a soluble to a solid or gelled state without exogenous agents, inherent biodegradability, and remarkable molecular flexibility. Unlike structural proteins such as collagen or fibrin, albumin undergoes solidification through subtle mechanisms of protein crosslinking rather than polymerization, enabling the formation of tunable networks suitable for biomedical applications.
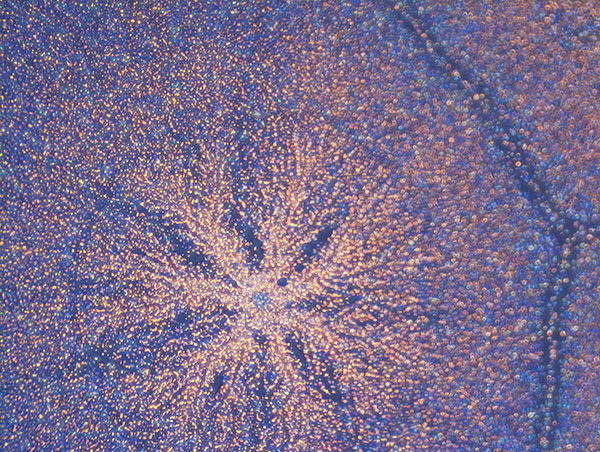
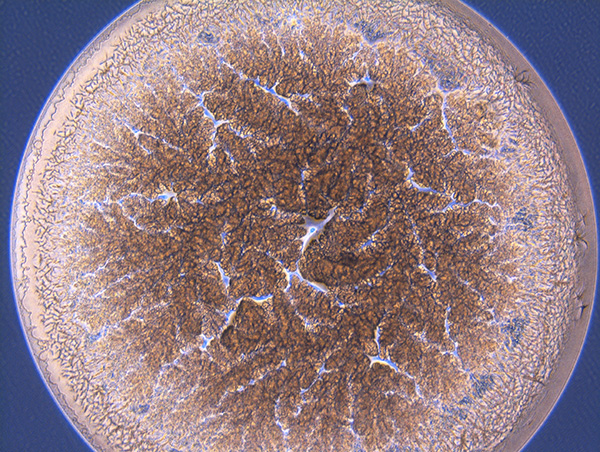
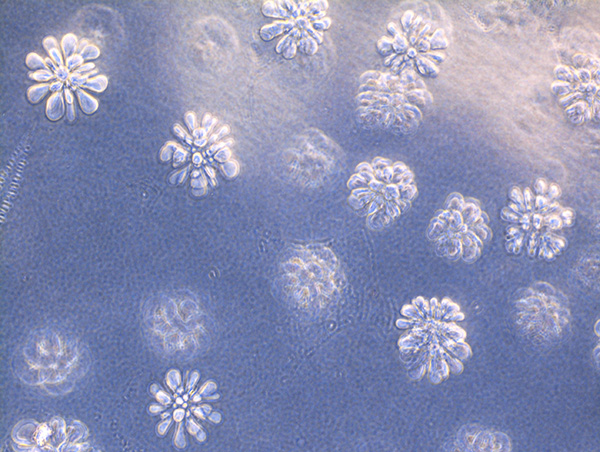
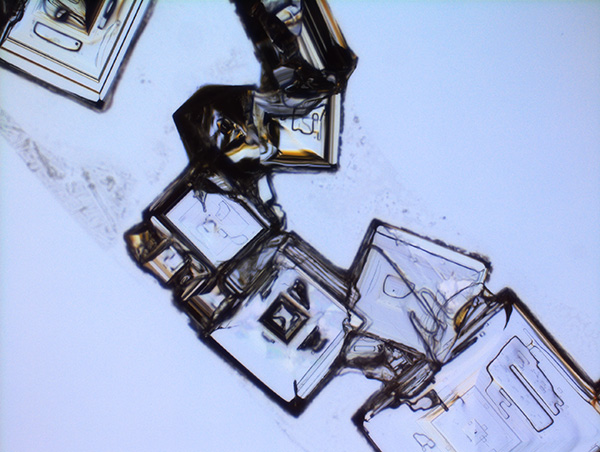
From Natural Biomatters to ALBUPAD Breakthrough Technology
Albumin remarkable properties become fully meaningful when albumin is transformed through a technology capable of precisely controlling its form, density, porosity, crosslinking structure, and degradation kinetics.
This is what the ALBUPAD technology achieves in a cost- and eco-efficient way: it converts native albumin into a highly functional biomaterial through a solvent-free process. By exploiting the intrinsic physicochemical behavior of albumin, ALBUPAD enables the production of solid matrices, films, microdiscs, and three-dimensional constructs that are uniform, stable, reproducible, and inherently biocompatible. The process provides levels of structural control that were previously unattainable for a non-modified protein-based material.
By combining biological safety, structural programmability, controlled diffusion capacity, predictable biodegradation, and adaptability to a wide range of medical applications, albumin shaped through ALBUPAD technology emerges as a new category of biomaterial: natural, programmable, and functionally exceptional. This positions it as a promising platform for drug delivery, resorbable implants, accidental or surgical wound healing, and advanced tissue engineering.

OUR CHALLENGE
Sustained delivery of Active Pharmaceutical Ingredients (APIs)
APPLICATION + API


Treatment optimization -> Bioavailability
Improves the integration of treatment into patients’ daily lives -> therapeutic adherence
THE NEED
CURRENT SOLUTION : Synthetic polymers/plastics
• Release with burst effect
• Incompatible with the loading of many APIs
Peptides & therapeutic proteins, small hydrophilic molecules

OUR SOLUTION
Native albumin-based materials for sustained drug delivery
Injectable microparticles
Native human albumin

Most abundant plasma protein
• Biocompatible, biodegradable, non-immunogenic
• Available raw materials (GMP grade)
• Natural carrier protein (established therapeutic use for drug delivery)
 Non-denaturing process for the production of protein-based materials
Non-denaturing process for the production of protein-based materials

Patent : European patent (2019), PCT extension (2020), National phases Expertise
Material Ι Process Ι Application
Exclusive licensing agreement concluded with Conectus 2024
OUR TECHNOLOGY
 Non-denaturing process for the preparation of protein-based materials
Non-denaturing process for the preparation of protein-based materials



Albumin + Salt
Evaporation 37°C
Washing (water)
100% Native Albumin
EXPERTISE
Formulation development Drug encapsulation





MEMBRANES
COMPACT MATERIALS
SPONGES
EXTRUDABLE MATERIALS
MICROPARTICLES
