Strong value. Dual model. Scalable impact.
Though built for partnerships, Albupad is powered for products.
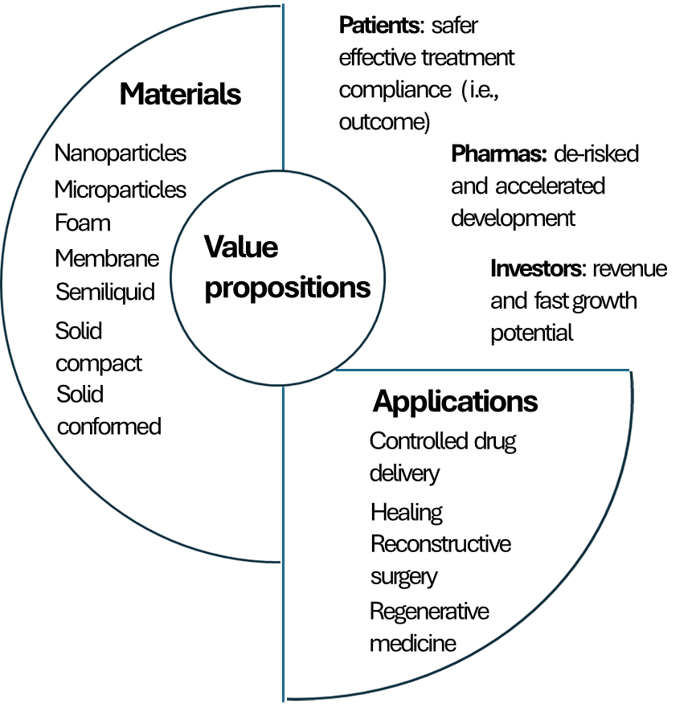
ALBUPAD delivers strong value propositions through a dual business model combining strategic partnerships with a growing proprietary pipeline. This approach accelerates market access, diversifies revenue streams, and maximizes the platform’s clinical and commercial impact across multiple therapeutic areas and approaches.
ALBUPAD significantly de-risks pharmaceutical development by building on albumin — a well-characterized, clinically validated, and regulatorily familiar biomolecule with decades of safe use across multiple drug products. Its long history of approval by the FDA, EMA, and global agencies provides a uniquely strong foundation for accelerated development, predictable regulatory pathways, and reduced clinical uncertainty.
By leveraging the intrinsic properties of albumin – biocompatibility, biodegradability, tumor tropism, and inherent imaging visibility – ALBUPAD minimizes the need for synthetic targeting ligands, toxic excipients, or novel contrast agents. This biological familiarity translates into lower nonclinical burden, greater CMC predictability, and a smoother regulatory trajectory, compared to platforms relying on unproven or highly engineered materials.
A key advantage of ALBUPAD is its broad formulation flexibility: it can support generic drugs seeking differentiation, improved delivery, or lifecycle extension, as well as first-in-class and best-in-class innovative therapies requiring targeted or image-guided delivery. This dual applicability dramatically increases the platform’s reach and commercial relevance.
ALBUPAD offers a de-risked, regulatorily aligned, and highly versatile biomaterial platform that enables both generic optimization and frontier innovation – reducing time-to-clinic, expanding therapeutic opportunity, and strengthening the probability of clinical and commercial success.
